07 July 2025 | Monday | News
Abinopharm, Inc., an innovative nutritional and biopharmaceutical company based in Shelton, Connecticut, USA, is pleased to announce that its partner, EGT Synbio, headquartered in Shanghai, China, has successfully completed two human clinical trials evaluating the effects of oral L-ergothioneine (Dr.Ergo®) on skin health. Abinopharm, Inc. is the exclusive U.S. collaborator and distributor for EGT Synbio’s premium L-ergothioneine product, Dr.Ergo®.
L-Ergothioneine was first isolated in 1909 by French chemist Charles Tanret from the ergot fungus (Claviceps purpurea), giving the compound its name: “ergo” from ergot and “thioneine” denoting a sulfur-containing compound. Although ergothioneine is found in many plants and animals, it cannot be synthesized by them and must be acquired from fungi—especially mushrooms—and certain bacteria. In humans, dietary intake of mushrooms, particularly shiitake, maitake, and oyster mushrooms, which contain 10–13 mg/100 g dry weight, is the main source.
For many years, the biological significance of ergothioneine remained obscure. This changed in 2005 when Professor Günther Gündemann discovered the ergothioneine-specific transporter OCTN1 (SLC22A4), which facilitates its accumulation in nearly all human tissues, particularly in the bone marrow, liver, kidneys, red blood cells, brain, eyes, and skin.
Professor Barry Halliwell, a pioneer in the study of reactive oxygen species (ROS) and oxidative stress, later identified ergothioneine as one of the most potent natural antioxidants. He demonstrated its role in mitigating oxidative damage and inflammation—factors contributing to conditions such as neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s), cardiovascular disorders, and liver disease. Studies have also shown that ergothioneine has high bioavailability, a long half-life in the human body, and offers cellular protection—particularly within mitochondria—thereby helping maintain cognitive function, reduce inflammation, and support healthy longevity.
In 2018, renowned biochemist Professor Bruce Ames proposed that ergothioneine be classified as a “longevity vitamin”—a micronutrient not essential for immediate survival but vital for long-term health and healthy aging. He suggested that low levels of ergothioneine may accelerate aging and increase the risk of chronic diseases.
A Nutrient for Radiant and Resilient Skin
Ergothioneine is a multifunctional skincare nutrient known for its antioxidant power, anti-inflammatory effects, and UV protection. It protects skin cell components—lipids, DNA, and proteins—from oxidative damage and premature aging, helps prevent photoaging (e.g., sunspots, collagen breakdown), soothes redness and inflammation (e.g., acne, eczema, sensitive skin), promotes even skin tone and radiance, supports collagen synthesis and elasticity, and enhances skin hydration.
While ergothioneine has been used topically in cosmetic products for years, a 2024 Japanese clinical study confirmed its effectiveness as an oral "beauty-from-within" ingredient. In this study, participants who consumed a hiratake (oyster mushroom) tablet containing 25 mg of ergothioneine daily experienced significant improvements in facial skin moisture and overall skin condition (Frontiers in Medicine, 2024).
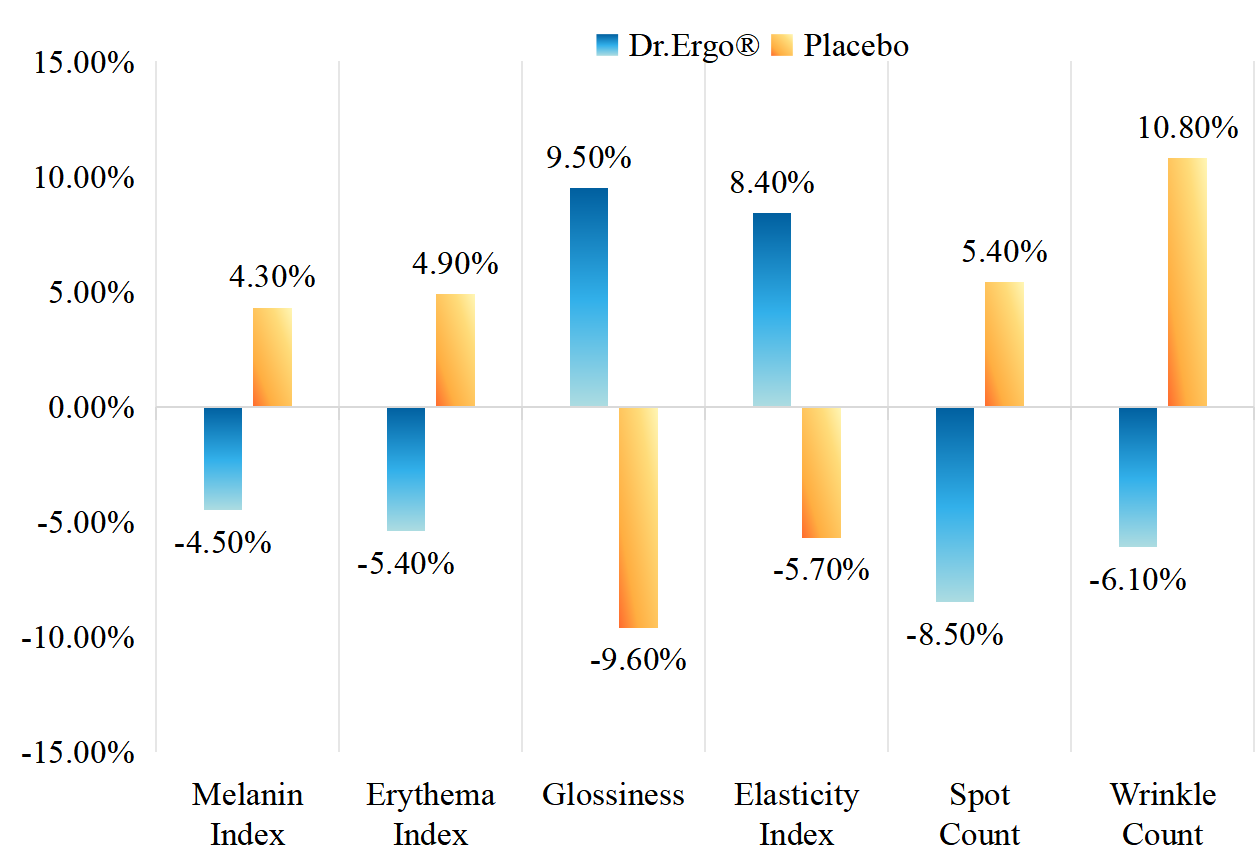
New Clinical Results with Dr.Ergo®
Abinopharm and EGT Synbio now report results from two human clinical trials evaluating the efficacy of Dr.Ergo® L-ergothioneine for skin health:
Quality and Safety Commitment
Dr.Ergo® is produced using a patented full enzymatic fermentation process (EP 4520819A1) under strict cGMP standards. It has been granted U.S. FDA GRAS (Generally Recognized as Safe) status, NSF GMP certification, HACCP compliance, and HALAL certification. The product adheres to ICH quality guidelines, with purity and optical purity >99.9%. All impurities, heavy metals, and microbial levels are controlled within USP limits.
© 2026 Biopharma Boardroom. All Rights Reserved.