03 February 2026 | Tuesday | News
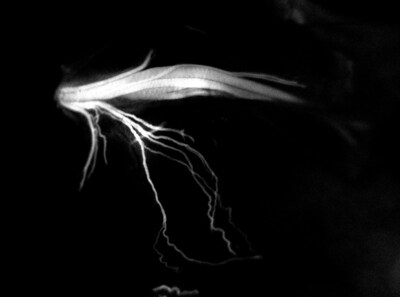
A new roadmap to surgery - IND-approved LGW16-03, also known as NerveTrace Dx, enables real-time visualization of complex nerve anatomy so surgeons can cut by color to spare critical nerves and improve patient outcomes.
Trace Biosciences, Inc., a clinical-stage biotechnology company developing nerve-targeted imaging agents, announced that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for LGW16-03, the company's first nerve-specific fluorescent imaging agent. The IND clearance enables Trace to initiate first-in-human clinical studies evaluating the safety and intraoperative performance of LGW16-03 in surgical settings.
LGW16-03 is designed to selectively bind peripheral nerves and emit a near-infrared fluorescent signal, allowing surgeons to visualize critical nerve structures in real time during surgery even when buried beneath tissues. Accidental nerve injury remains a significant and under-addressed cause of surgical complications across procedures such as prostatectomy, orthopedic surgery, colorectal surgery, and head and neck surgery. These injuries can result in chronic pain, incontinence, numbness, sexual dysfunction, voice loss, or other permanent loss of function – outcomes that profoundly impact patients' quality of life.
"This IND clearance is a major milestone for Trace and validates more than a decade of scientific work focused on making nerves visible to surgeons," said Connor Barth, Ph.D., Co-Founder and CEO of Trace Biosciences. "Despite the prevalence and severity of nerve injury, surgeons still lack approved tools to reliably visualize nerves in real time. We believe LGW16-03 has the potential to fundamentally improve surgical safety and patient outcomes."
Trace plans to initiate its Phase I clinical study later this year, initially evaluating safety and feasibility in patients undergoing orthopedic surgery. Following successful early clinical studies, the company intends to expand development across multiple surgical indications where nerve injury risk is high.
"Surgeons routinely operate near critical nerves with limited to no direct visibility, relying largely on anatomical knowledge and estimated location," said Nirmish Singla, MD, MSCS, FACS, urologic surgeon at Johns Hopkins Medicine and clinical advisor to Trace. "A real-time nerve imaging agent like LGW16-03 could meaningfully reduce avoidable nerve injuries and change how many common procedures are performed."
The IND clearance represents the first clinical program within Trace's broader nerve-targeted platform, which the company is advancing toward applications in fluorescence-guided surgery, nerve repair or stimulation guidance, and diagnostic imaging.
© 2026 Biopharma Boardroom. All Rights Reserved.