19 February 2026 | Thursday | News
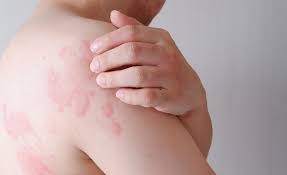
Novartis announced positive topline results from its pivotal Phase III RemIND trial of oral remibrutinib in chronic inducible urticaria (CIndU)1. The primary endpoint was met for the three most prevalent types of CIndU: symptomatic dermographism, cold urticaria and cholinergic urticaria, achieving significantly higher complete response rates versus placebo at Week 121. These data represent an important advance in the treatment of CIndU, demonstrating the potential of remibrutinib to be the first targeted therapy for CIndU and address a major unmet need.
“The positive RemIND trial results across three different types of CIndU underscore the potential of oral remibrutinib to achieve complete symptom relief for people living with CIndU and build on its recent FDA approval in chronic spontaneous urticaria (CSU),” said Angelika Jahreis, Global Head, Immunology Development, Novartis. “Today’s findings reinforce that remibrutinib could be the first targeted therapy to improve spontaneous and inducible forms of chronic urticaria, helping address a major gap in care for people living with these conditions.”
Novartis has submitted a supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) seeking approval of remibrutinib for the treatment of symptomatic dermographism, the most prevalent type of CIndU. In the coming months, the full data set will be submitted to health authorities globally, and the RemIND trial findings will be presented in upcoming medical congresses.
© 2026 Biopharma Boardroom. All Rights Reserved.